Table Of Elements Noble Gases: The Silent Stars Of The Periodic Table
Hey there science lovers! If you're diving into the world of chemistry, then you've probably come across the table of elements noble gases. These elements are like the quiet, cool kids in the periodic table who don't really interact much with others. But don't let their laid-back attitude fool you. They're actually super important and play a critical role in various scientific applications. Today, we're gonna dive deep into the fascinating world of noble gases and uncover why they're such a big deal in the world of chemistry.
Think about it like this: noble gases are like the zen masters of the periodic table. They're calm, collected, and completely at peace with themselves. Unlike other elements that are always trying to bond and react, noble gases prefer to keep to themselves. This unique characteristic makes them incredibly useful in various industries, from lighting to medical imaging. So, if you're ready to get nerdy about noble gases, buckle up because we're about to take a deep dive into their world!
Before we jump into the nitty-gritty details, let's quickly talk about why understanding noble gases is essential. Whether you're a student studying chemistry, a scientist exploring new frontiers, or just someone curious about the building blocks of our universe, noble gases have something to offer. They're not just random elements on the periodic table; they're keys to unlocking some pretty awesome scientific discoveries. So, let's get started and see what makes these gases so special.
- Explore Camilla Araujos Content Videos Photos On Erome
- Top Ullu Web Series Mustwatch List Where To Stream
What Are Noble Gases Anyway?
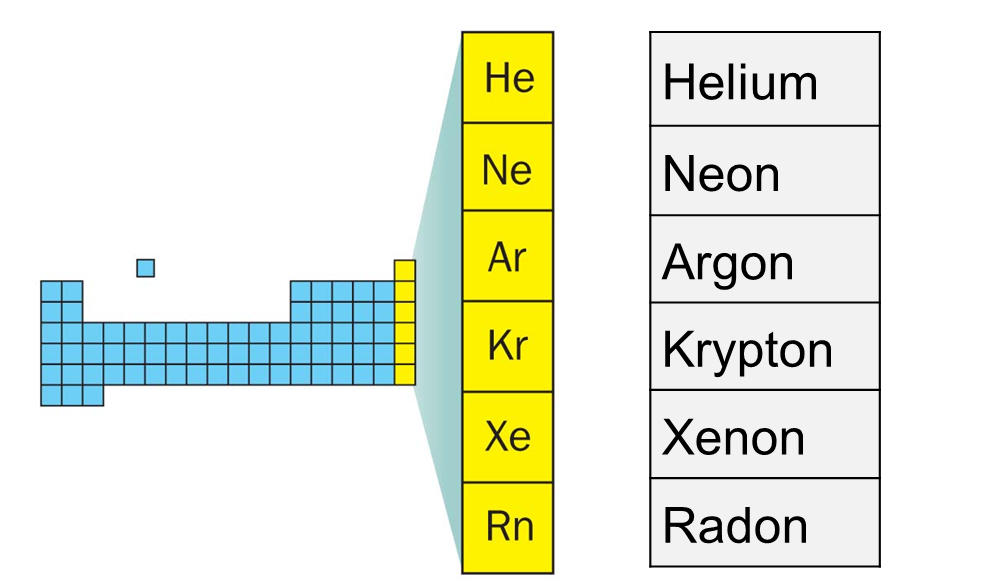
Alright, let's break it down. Noble gases are a group of chemical elements found in Group 18 of the periodic table. They're also known as inert gases because of their tendency to remain unreactive under most conditions. This group includes helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). What makes them stand out is their full outer electron shell, which gives them stability and a reluctance to form compounds with other elements. Basically, they're the chill ones in the periodic table who don't feel the need to mingle.
Why Are They Called Noble?
Now, you might be wondering why they're called "noble." Well, the term "noble" comes from the idea that these gases are aristocrats among elements. They don't mix with the common folk, so to speak. Their reluctance to react with other elements gives them an air of exclusivity, much like royalty who don't get involved in the affairs of ordinary people. This characteristic is what makes them so fascinating to scientists and researchers around the world.
The Periodic Table: Where It All Begins
The periodic table is like the family tree of elements, and noble gases hold a special place in it. They're located in the far-right column, also known as Group 18. This positioning isn't random; it reflects their unique properties and behavior. Unlike their neighbors, who are always trying to bond and react, noble gases prefer to keep their distance. It's like they have their own VIP section in the periodic table where they can relax and be themselves.
How Do Noble Gases Fit Into the Periodic Table?
When you look at the periodic table, you'll notice that noble gases are the only group with a full outer electron shell. This means they have a complete set of electrons in their outermost energy level, which makes them incredibly stable. Think of it like a house with all its windows and doors closed and locked. It's secure, self-contained, and doesn't need anything else. This stability is what gives noble gases their inert nature and makes them so useful in various applications.
The Noble Gas Family: Meet the Members
Let's take a closer look at the members of the noble gas family and what makes each one unique. Here's a quick rundown:
- Helium (He): The party gas that makes balloons float and voices sound funny.
- Neon (Ne): The bright light behind those colorful signs you see in cities.
- Argon (Ar): The most abundant noble gas in the Earth's atmosphere.
- Krypton (Kr): The gas that gives us those cool glowing lights in sci-fi movies.
- Xenon (Xe): Used in medical imaging and high-intensity lamps.
- Radon (Rn): A radioactive gas that can pose health risks if not handled properly.
Each of these gases has its own set of properties and applications, making them indispensable in various fields.
Interesting Facts About Noble Gases
Did you know that noble gases are used in everything from lighting to space exploration? Here are a few fun facts:
- Helium is the second most abundant element in the universe after hydrogen.
- Neon lights were first introduced in Paris in 1910 and quickly became a symbol of modernity.
- Argon is used in double-glazed windows to improve insulation and energy efficiency.
- Krypton is used in energy-efficient windows and high-performance lighting.
- Xenon is used in medical imaging and as an anesthetic in certain procedures.
- Radon is a naturally occurring gas that can accumulate in buildings and pose health risks if not monitored.
Applications of Noble Gases
Noble gases may be unreactive, but they're far from useless. In fact, they have a wide range of applications across various industries. From lighting to medical imaging, these gases play a crucial role in modern technology. Let's take a look at some of the most common applications:
Lighting and Displays
One of the most well-known applications of noble gases is in lighting and displays. Neon lights, for example, are made by passing an electric current through a tube filled with neon gas, producing a bright orange-red glow. Similarly, argon is used in fluorescent lights to stabilize the discharge and improve efficiency. Krypton and xenon are also used in high-intensity discharge lamps, providing bright and long-lasting illumination.
Medical Imaging and Anesthesia
In the medical field, noble gases are used in various imaging techniques, such as MRI and CT scans. Xenon, in particular, is used as an anesthetic in certain procedures due to its non-toxic and non-reactive nature. It's also used in hyperpolarized MRI, where it enhances the visibility of certain tissues and organs.
The Science Behind Noble Gases
Now, let's dive into the science behind noble gases. What makes them so unique and why do they behave the way they do? It all comes down to their electron configuration. Noble gases have a full outer electron shell, which gives them a stable and unreactive nature. This stability is what makes them so useful in various applications.
Why Are Noble Gases Inert?
The inertness of noble gases is due to their electron configuration. In a neutral atom, the number of protons equals the number of electrons. Noble gases have a complete set of electrons in their outermost energy level, which makes them stable and resistant to chemical reactions. This is why they're often referred to as inert gases.
Environmental Impact and Safety
While noble gases are generally safe and non-toxic, there are some environmental and safety considerations to keep in mind. For example, radon is a naturally occurring radioactive gas that can accumulate in buildings and pose health risks if not properly managed. It's important to monitor radon levels in homes and workplaces to ensure safety.
How to Safely Handle Noble Gases
When working with noble gases, it's important to follow proper safety protocols. While most noble gases are non-toxic, they can still pose risks if not handled properly. For example, inhaling large amounts of helium can lead to asphyxiation due to oxygen displacement. Always ensure proper ventilation and follow safety guidelines when working with noble gases.
Future Prospects and Research
The study of noble gases is an ongoing field of research, with new discoveries and applications emerging all the time. Scientists are exploring the potential of noble gases in areas such as quantum computing, space exploration, and medical diagnostics. As our understanding of these elements grows, so too does their potential to revolutionize various industries.
What's Next for Noble Gases?
With advancements in technology and research, the future of noble gases looks bright. From improving energy efficiency in lighting to enhancing medical imaging techniques, these gases continue to play a crucial role in modern science. As we continue to explore their properties and applications, we may discover even more ways to harness their power and potential.
Conclusion: Why Noble Gases Matter
In conclusion, the table of elements noble gases may seem unassuming at first glance, but they're actually incredibly important. Their unique properties and behavior make them indispensable in various fields, from lighting to medicine. Understanding noble gases and their applications can open up new possibilities for scientific discovery and technological advancement. So, the next time you see a neon sign or use a helium balloon, take a moment to appreciate the noble gases that make it all possible.
Now, it's your turn! If you found this article helpful or have any questions about noble gases, feel free to leave a comment below. And don't forget to share this article with your friends and family. Who knows? You might just inspire someone to become a chemistry enthusiast!
Table of Contents
- What Are Noble Gases Anyway?
- The Periodic Table: Where It All Begins
- The Noble Gas Family: Meet the Members
- Applications of Noble Gases
- The Science Behind Noble Gases
- Environmental Impact and Safety
- Future Prospects and Research
- Conclusion: Why Noble Gases Matter


Detail Author:
- Name : Mr. Demarcus Sauer DDS
- Username : bhudson
- Email : schulist.alford@hotmail.com
- Birthdate : 1985-10-06
- Address : 8249 Gennaro Courts Rafaelabury, DC 77798-8031
- Phone : +1.678.290.1734
- Company : Gutmann, Hickle and Bashirian
- Job : Oil and gas Operator
- Bio : Est voluptas officia molestiae eos pariatur et. Ab doloribus odit alias nisi perspiciatis ea sed. Odit eum voluptatum qui sint.
Socials
linkedin:
- url : https://linkedin.com/in/clare_dev
- username : clare_dev
- bio : Tempore ab quasi expedita dolor.
- followers : 1905
- following : 564
instagram:
- url : https://instagram.com/clarebuckridge
- username : clarebuckridge
- bio : Hic aut sed aperiam dicta et aut harum. Cum non molestiae beatae. Dignissimos sunt ex quis sit.
- followers : 3616
- following : 2855
facebook:
- url : https://facebook.com/buckridge2021
- username : buckridge2021
- bio : Laboriosam voluptas qui vitae similique repellat deleniti.
- followers : 3692
- following : 2972