What Temp Is Boiling Water? The Ultimate Guide To Understanding Boiling Point
Boiling water might sound simple, but there's more to it than meets the eye. Have you ever wondered what temp is boiling water? It's not just about turning liquid into vapor; it's a fascinating process that involves physics, chemistry, and even altitude. Whether you're cooking pasta, making tea, or conducting a science experiment, understanding the boiling point of water is crucial. So, buckle up and let's dive deep into the world of boiling water!
You might think boiling water is as straightforward as turning on the stove, but trust me, there's a lot more going on beneath the surface. The boiling point of water isn't a fixed number—it changes depending on factors like pressure, altitude, and even impurities in the water. In this article, we'll break it all down for you in a way that's easy to understand yet packed with valuable insights.
From the science behind boiling water to practical tips for everyday use, we've got you covered. Whether you're a curious cook, a science enthusiast, or just someone who wants to impress friends with random trivia, this guide is here to help. So, let's get started and uncover the mysteries of boiling water!
Understanding the Basics: What Temp is Boiling Water?
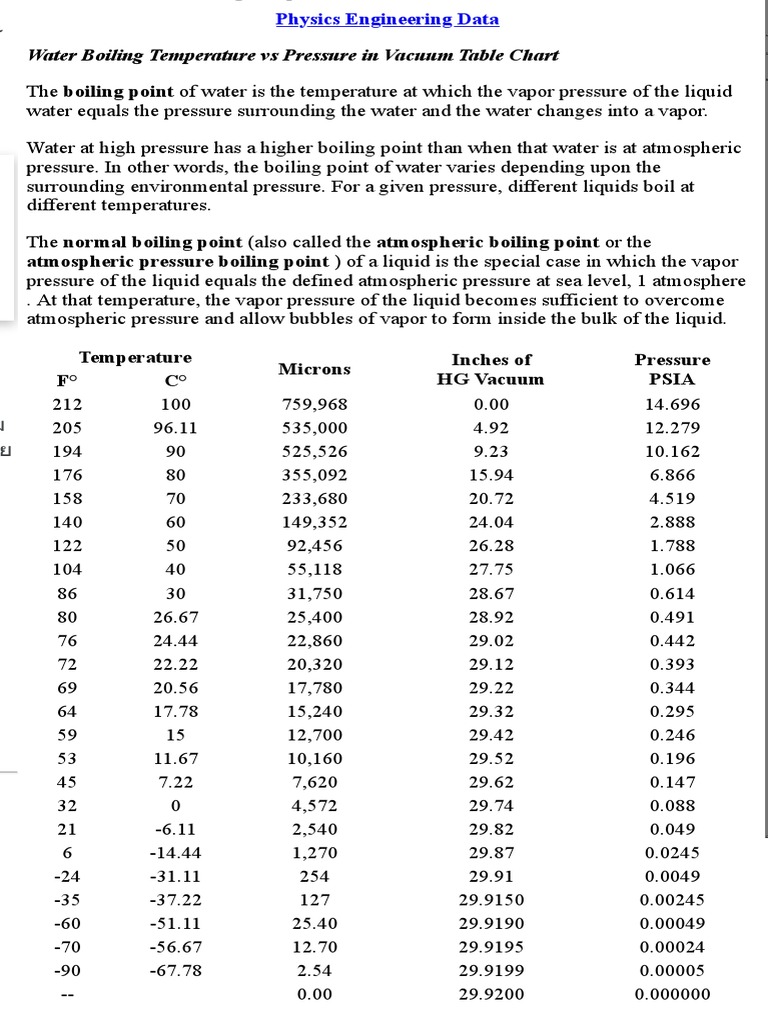
Let's start with the basics. The boiling point of water under standard atmospheric pressure (at sea level) is 100°C or 212°F. This is the temp where water transitions from liquid to gas. But here's the catch—this temp isn't constant everywhere. Factors like altitude and atmospheric pressure play a huge role in altering the boiling point.
What Happens When Water Boils?
When water reaches its boiling point, it starts to form bubbles of vapor. These bubbles rise to the surface, creating the familiar sight of boiling water. But why does this happen? It's all about energy. As heat is applied, the water molecules gain energy and start moving faster. Eventually, they have enough energy to break free from the liquid state and enter the gas phase.
Here are some key points to remember:
- Boiling is a phase transition from liquid to gas.
- The boiling point depends on external factors like pressure.
- At higher altitudes, water boils at a lower temp due to reduced atmospheric pressure.
Factors That Affect the Boiling Point of Water
While 100°C (212°F) is the standard boiling point of water, several factors can influence this temp. Let's take a closer look at what affects the boiling point and why it matters.
Altitude and Atmospheric Pressure
As you go higher in altitude, atmospheric pressure decreases. This means water boils at a lower temp because there's less pressure pushing down on the liquid. For example, at an altitude of 5,000 feet, water boils at around 94.5°C (202°F). This is why cooking instructions often vary depending on your location.
Impurities in Water
Believe it or not, adding impurities to water can change its boiling point. For instance, adding salt to water increases the boiling point slightly. This is due to a phenomenon called boiling point elevation, where dissolved substances raise the temp at which water boils. So, if you're boiling pasta, adding a pinch of salt doesn't just enhance flavor—it also affects the cooking process!
Practical Applications: Why Knowing the Boiling Point Matters
Understanding the boiling point of water isn't just for scientists or chefs. It has real-world applications that affect our daily lives. Whether you're camping at high altitudes or troubleshooting a cooking recipe, knowing the boiling temp can make a big difference.
Cooking at High Altitudes
If you live in a mountainous region, you've probably noticed that food takes longer to cook. This is because water boils at a lower temp, which slows down the cooking process. To compensate, you might need to increase cooking times or use pressure cookers, which trap steam and increase the internal pressure, allowing water to boil at a higher temp.
Boiling Water for Safety
Boiling water is one of the most effective ways to purify it and make it safe to drink. The high temp kills bacteria, viruses, and other harmful microorganisms. This is especially important when traveling to areas with questionable water sources or during emergencies when clean water isn't readily available.
Science Behind Boiling Water: A Deeper Dive
For those of you who want to geek out about the science, let's explore the physics and chemistry behind boiling water. It's not just about heat—it's about molecular interactions and energy transfer.
Heat Transfer and Energy
When you heat water, you're essentially transferring energy to the molecules. This energy causes the molecules to move faster and collide with each other more frequently. As the temp rises, the collisions become more energetic, eventually leading to the formation of vapor bubbles. This process requires a specific amount of energy, known as the latent heat of vaporization.
Phase Transitions and Thermodynamics
Boiling is a phase transition, which means it involves a change in the state of matter. According to thermodynamics, this transition occurs when the vapor pressure of the liquid equals the external pressure. At this point, the liquid begins to vaporize, and bubbles form throughout the liquid, not just at the surface.
Boiling Water in Everyday Life: Tips and Tricks
Now that we've covered the science and factors affecting boiling water, let's talk about how you can apply this knowledge in your daily life. Here are some practical tips to help you make the most of boiling water:
Speeding Up the Boiling Process
If you're in a hurry, there are a few tricks to speed up the boiling process. Using a lid on your pot can trap heat and reduce the time it takes for water to boil. Additionally, starting with hot tap water instead of cold can shave off a few minutes. However, keep in mind that these methods might not work as well at high altitudes.
Boiling Water for Cooking
Cooking with boiling water is a staple in many cuisines. Whether you're making pasta, rice, or vegetables, the temp of the water plays a crucial role. For example, pasta should be cooked in rapidly boiling water to ensure it cooks evenly and doesn't stick together. On the other hand, some delicate foods, like eggs, benefit from a gentle simmer rather than a rolling boil.
Common Myths About Boiling Water
There are plenty of myths surrounding boiling water, and it's time to set the record straight. Here are a few common misconceptions and the truth behind them:
Myth: Adding Salt Makes Water Boil Faster
Fact: Adding salt to water slightly increases the boiling point, but it doesn't make the water boil faster. The difference is negligible unless you're using a large amount of salt.
Myth: Boiling Water Kills All Germs
Fact: While boiling water is highly effective at killing most bacteria and viruses, it may not eliminate all contaminants, especially chemical pollutants. For complete purification, consider using additional methods like filtration or chemical treatments.
Boiling Water Around the World
Boiling water isn't just a scientific or culinary phenomenon—it's a cultural one too. Different regions and cuisines have unique ways of using boiling water, and these traditions reflect the local environment and resources.
Traditional Uses of Boiling Water
In many cultures, boiling water has been used for centuries as a method of cooking, purification, and even medicine. From the tea ceremonies of Japan to the soups of Europe, boiling water plays a central role in culinary traditions worldwide.
Future Trends: Innovations in Boiling Water Technology
Technology is constantly evolving, and boiling water is no exception. Modern innovations like induction cooktops and instant hot water dispensers are changing the way we heat water. These advancements not only make boiling water faster and more efficient but also reduce energy consumption and environmental impact.
Conclusion: What Temp is Boiling Water?
In conclusion, the temp at which water boils isn't a one-size-fits-all answer. It depends on factors like altitude, atmospheric pressure, and impurities in the water. Understanding these factors can help you make informed decisions, whether you're cooking, purifying water, or conducting experiments.
So, the next time someone asks you, "What temp is boiling water?" you'll have a wealth of knowledge to share. And remember, if you found this article helpful, don't forget to leave a comment or share it with your friends. Together, let's keep the conversation boiling!
Daftar Isi:
- Understanding the Basics: What Temp is Boiling Water?
- Factors That Affect the Boiling Point of Water
- Practical Applications: Why Knowing the Boiling Point Matters
- Science Behind Boiling Water: A Deeper Dive
- Boiling Water in Everyday Life: Tips and Tricks
- Common Myths About Boiling Water
- Boiling Water Around the World
- Future Trends: Innovations in Boiling Water Technology
- Conclusion: What Temp is Boiling Water?


Detail Author:
- Name : Arnulfo Jacobi
- Username : june.rau
- Email : kautzer.elizabeth@stroman.com
- Birthdate : 1988-02-11
- Address : 27329 Watsica Overpass New Dorthahaven, MI 24875-8288
- Phone : (785) 915-5633
- Company : Friesen-Nader
- Job : Photoengraving Machine Operator
- Bio : Et officiis aut nulla repellendus. Modi maxime consequuntur hic pariatur. Tempore id recusandae dolorum optio aut sint pariatur quo. Error alias ut veritatis porro et molestias.
Socials
twitter:
- url : https://twitter.com/mitchell_raynor
- username : mitchell_raynor
- bio : Aspernatur a aut culpa est iusto ipsa. Eius aut sapiente nostrum. Fuga ut ad repellendus quis. Cum dolor quia cum eum reiciendis iure.
- followers : 6187
- following : 1427
tiktok:
- url : https://tiktok.com/@raynorm
- username : raynorm
- bio : Enim fugiat in iusto sit quos consequuntur eos.
- followers : 5910
- following : 2506
linkedin:
- url : https://linkedin.com/in/raynorm
- username : raynorm
- bio : Sed in eveniet eaque repellendus enim fugit.
- followers : 5839
- following : 2348
facebook:
- url : https://facebook.com/raynorm
- username : raynorm
- bio : Dolores vel sit cupiditate ipsum optio excepturi qui.
- followers : 3397
- following : 1694
